Our Research
The Development of Novel Treatments for Pancreatic Cancer and COVID infections
- Our research demonstrates that cancer treatments such as surgical removal of a primary tumor triggers a distinct inflammatory response expressed within a narrow and predictable window of time after treatment. This response has been shown to foster cancer stem cell enrichment by triggering a process called epithelial-mesenchymal transition, or EMT. This process can foster metastasis as well as drug resistance.
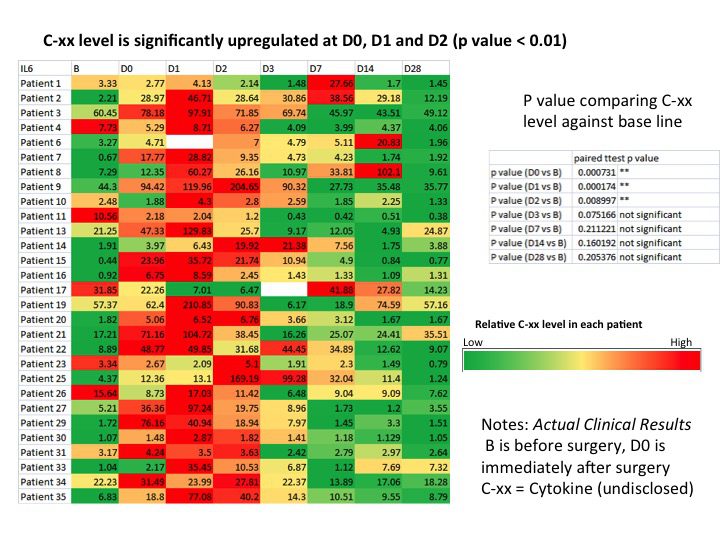
- A post treatment wound healing response includes the significant up regulation of several distinct cytokine molecules detectable in the plasma of patients undergoing cancer treatments.
- Our research has confirmed that specific cytokines, in distinct combinations at concentration levels noted in patients’ plasma, foster rapid stem cell proliferation and epithelial mesenchymal transition (EMT) in cancer cells leading to stem cell enrichment and a drug-resistant phenotype.
- Preliminary results in patients undergoing chemotherapy and radiation therapy suggest a similar pattern of cytokine release triggered by these treatments.
- Comparison of anti-cancer therapies used before and after exposure to these molecules in vitro demonstrates that cancer stem cells can be killed much more effectively if treatments are administered at the time the cells are cycling, rendering normally treatment resistant cancer stem cells vulnerable to anti-cancer therapies during a narrow window of time.
Example of a Specific Cytokine Found to be Significantly Up Regulated After Cancer Surgery
THERAPEUTIC APPLICATIONS
- Knowing exactly when cancer stem cells proliferate allows optimization of conventional treatments, resulting in significantly improved effectiveness.
- Knowing which cytokine expressions are responsible for stem cell proliferation after upfront cancer therapies will become a key enabler for increasing the effectiveness of existing monoclonal antibody therapies for disrupting the influence of these molecules on a surviving cancer cell population
- Blocking these cytokines at the time of their upregulation after initial chemotherapy/radiation may prevent stem cell repopulation and the rapid development of drug resistance.
PANCREATIC CANCER RESEARCH
- Pancreatic cancer today has a case fatality rate of approximately 94% and a one-year survival rate of approximately 25%, with few effective treatment options available to patients with this dreaded diagnosis.
- It remains one of the most difficult malignancies to treat with few treatment options available and is predicted to be the second leading cause of cancer death in the next decade, after lung cancer.
- In collaboration with our research partners at the Dr. Szewczuk Laboratory at Queen's University in Kingston, Ontario, we have developed a novel treatment approach for metastatic pancreatic cancer that blocks a key enzyme, mammalian neuramindase-1, which we think plays a key role in the regulation of a number of cell surface receptors that are being triggered after cancer treatments like surgery and chemotherapy.
- In our published pre-clinical animal studies this novel treatment reduced the growth and metastatic spread of an implanted human pancreatic cancer in an animal model by approximately 90% compared to the current standard treatmentwith chemotherapy alone.
- Intriguingly, our novel treatment was also able to inhibit cancer stem cell enrichment and the associated development of resistance to chemotherapy by inhibition of the EMT process.
- These very promising preclinicalresults may provide a path forward in the treatment of this very difficult to treat human cancer.
- A human clinical trial for patients with metastatic pancreatic cancer is planned to start enrolling patients in 2025.
COVID RESEARCH
- ENCYT in collaboration with the Dr. Szewczuk Laboratory at Queens University, we have also discovered that the enzyme critical to pancreatic cancer growth is also important in regulating the receptor that the SARS-COV2 virus, or COVID virus, is using to enter the cell and infect the host. Blocking this enzyme could prove very effective in the treatment of severe COVID infections and we have applied for patent protection for this exciting discovery.
